EUFLEXXA has a demonstrated safety profile in all clinical trials2
EUFLEXXA patients experienced a low incidence of joint effusions2
AEs* in 12-Week Pivotal Trial vs Synvisc2,3
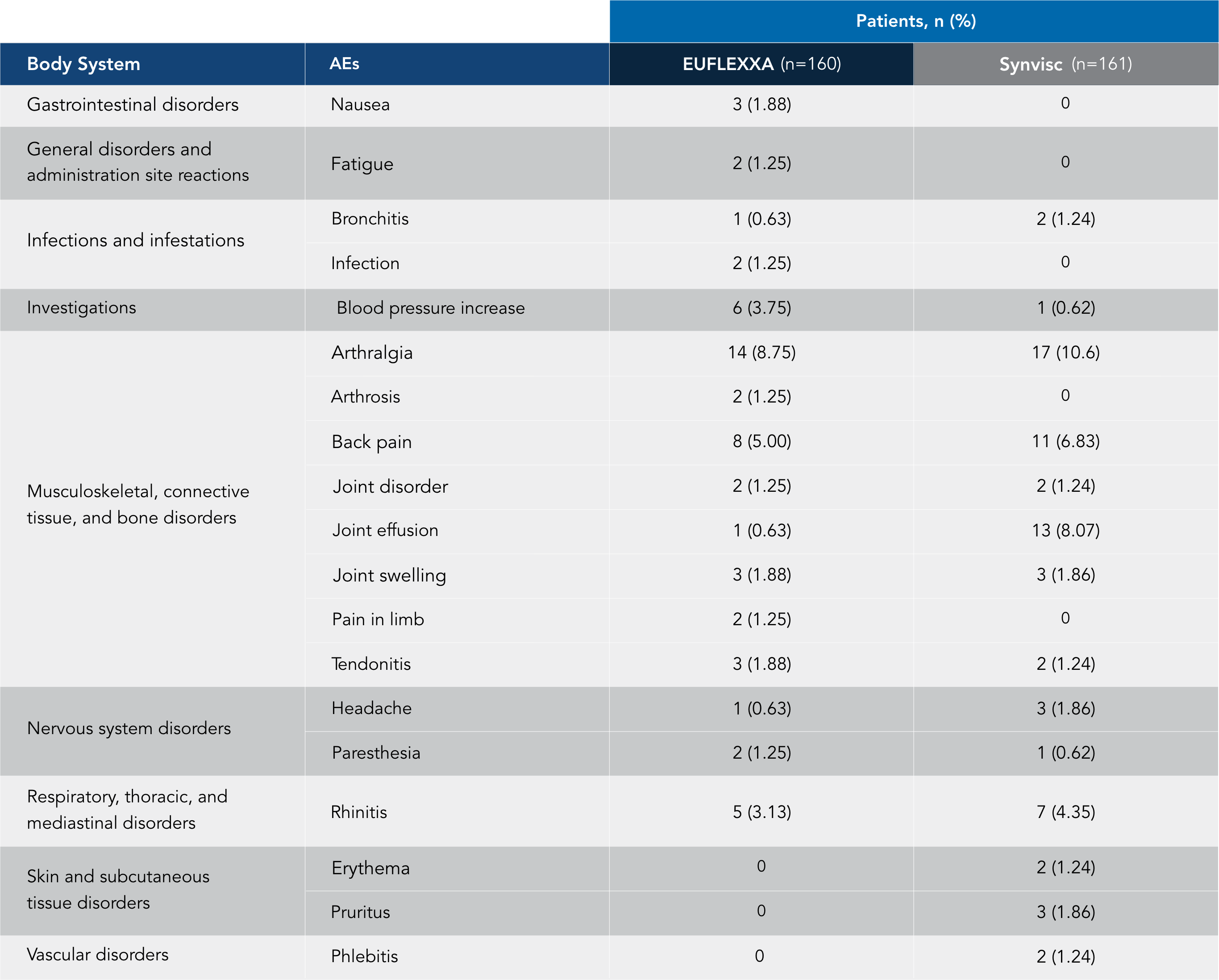
No reported AEs of pseudosepsis in this trial.
A total of 160 patients received 478 injections of EUFLEXXA. There were 27 reported AEs related to EUFLEXXA injections: arthralgia, 11 (6.9%); back pain, 1 (0.63%); blood pressure increase, 3 (1.88%); joint effusion, 1 (0.63%); joint swelling, 3 (1.88%); nausea, 1 (0.63%); paresthesia, 2 (1.25%); feeling of sickness from injection, 3 (1.88%); skin irritation, 1 (0.63%); and tenderness in study knee, 1 (0.63%). Four AEs were reported in the EUFLEXXA group for which the relationship to treatment was considered to be unknown: fatigue, 3 (1.88%) and nausea, 1 (0.63%).
AEs* in the FLEXX and FLEXX Extension Trials2
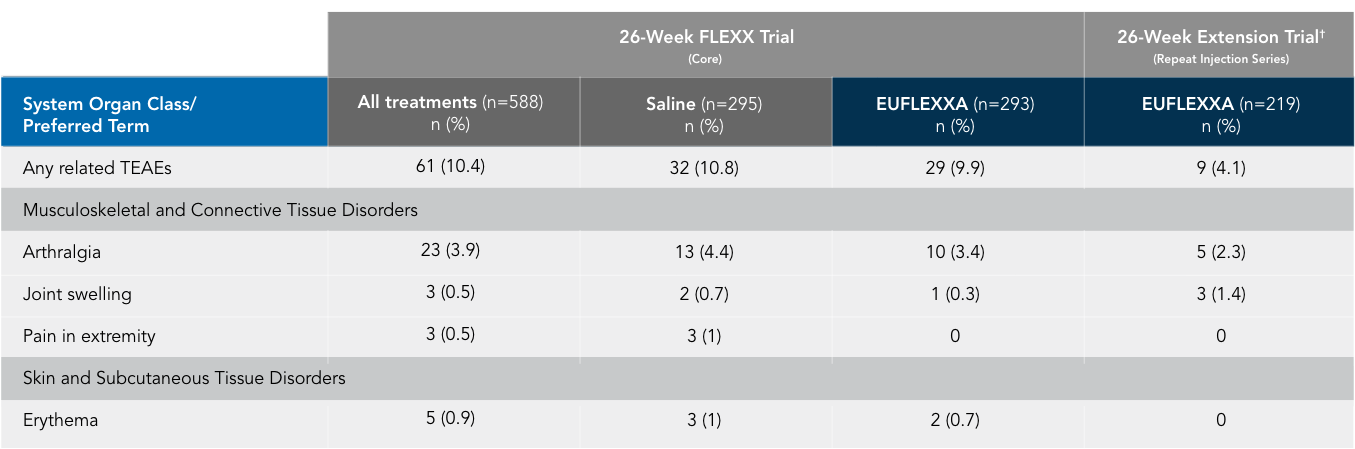
No cases of pseudosepsis were reported in the trial and extension.
*Preferred term with an incidence of >1 among treatment groups (safety population).
†TEAEs were reported for subjects who received EUFLEXXA in both the core and extension trials (219 of 433).
N=number of subjects in a given treatment group for the population analyzed; n=number of subjects reporting at least 1 AE within system organ class/preferred term; (%)=percent of subjects based on N. Note: Related AEs are those with trial device relationship classified as “certain,” “probably,” “possible,” or “unassessable.”

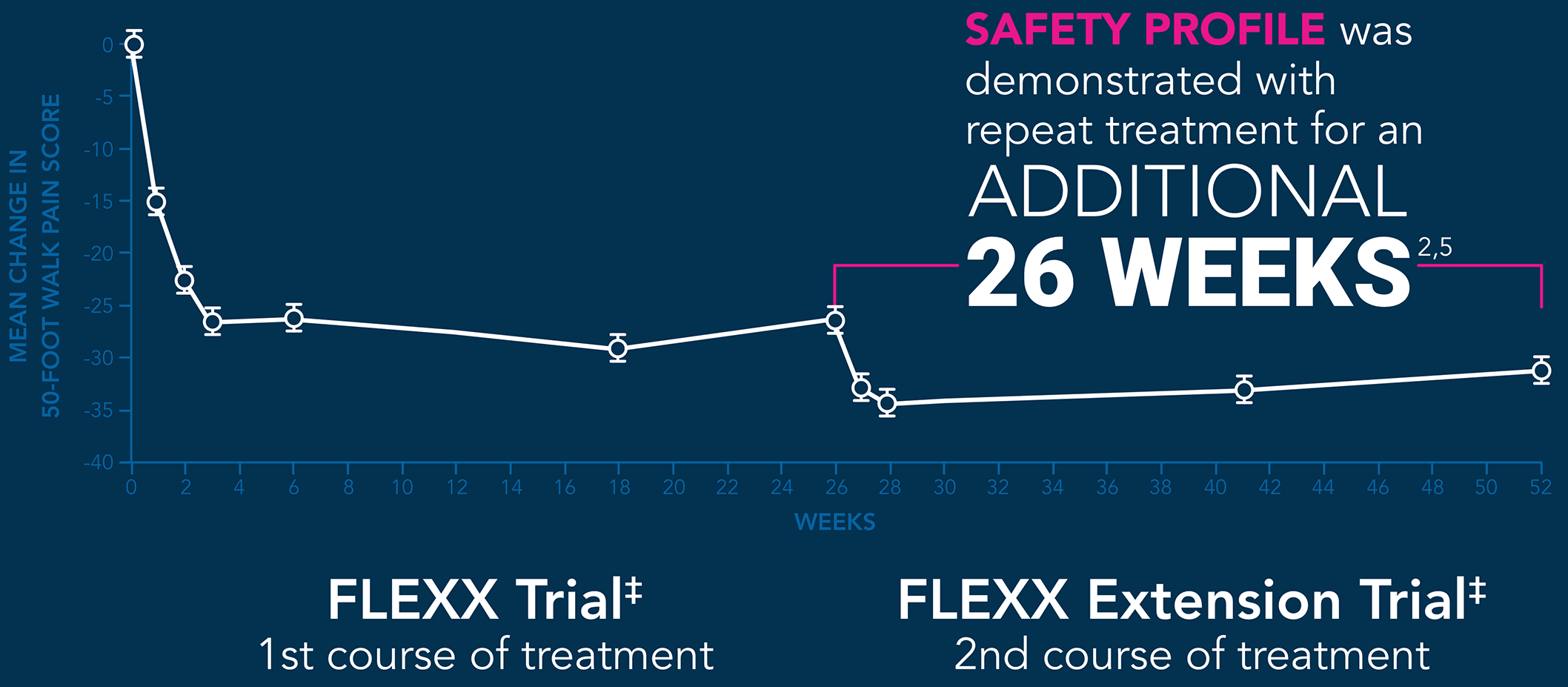
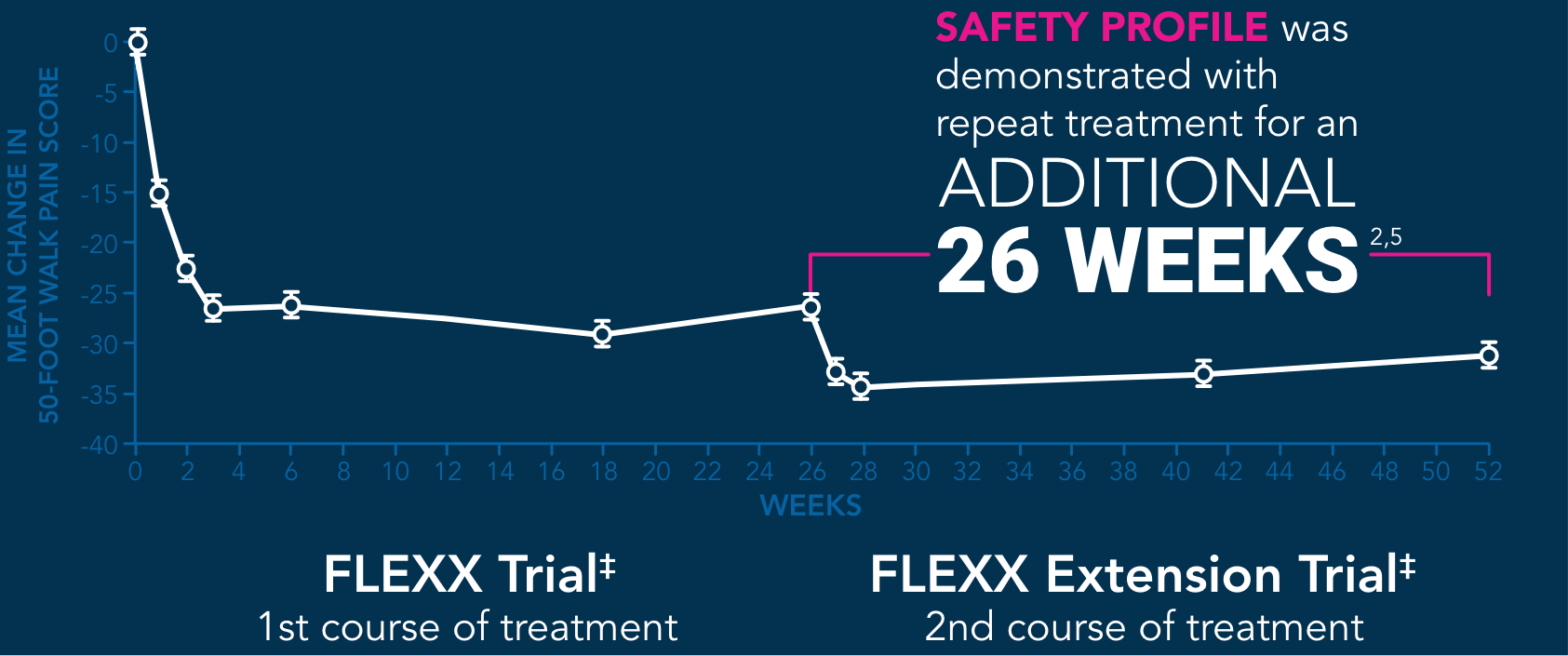
‡Only data points from EUFLEXXA patients are shown.
Dual-Acting Effects
Find out how EUFLEXXA may help your patients’ OA knee pain in two ways.
Discover The Effects- Rolling 12 month average of IQVIA claims data based on unique patients (December 2022).
- EUFLEXXA [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.
- Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(2):154-162.
- Altman RD, Rosen JE, Bloch DA, et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX Trial). Semin Arthritis Rheum. 2009;39(1):1-9.
- Altman RD, Rosen JE, Bloch DA, et al. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label Extension Study of the FLEXX Trial. Osteoarthritis Cartilage. 2011;19(10):1169-1175.
- Synvisc [package insert]. Cambridge, MA: Genzyme Corp; 2010.
- Yoshioka K, Katayama M, Nishiyama T, Harada K, Takeshita S, Kawamata Y. Biocompatibility study of different hyaluronan products for intra-articular treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2019;20(1):424.
