EUFLEXXA—the highest molecular weight of any linear HA, successfully binds to CD44 receptors2-11
Mechanical2-4
- Improves viscoelasticity
- Joint lubrication
- Cushioning
Physiologic2-5
- Disrupts the inflammatory cascade
- Reduces pain
- Protects cartilage
- Stimulates endogenous HA production
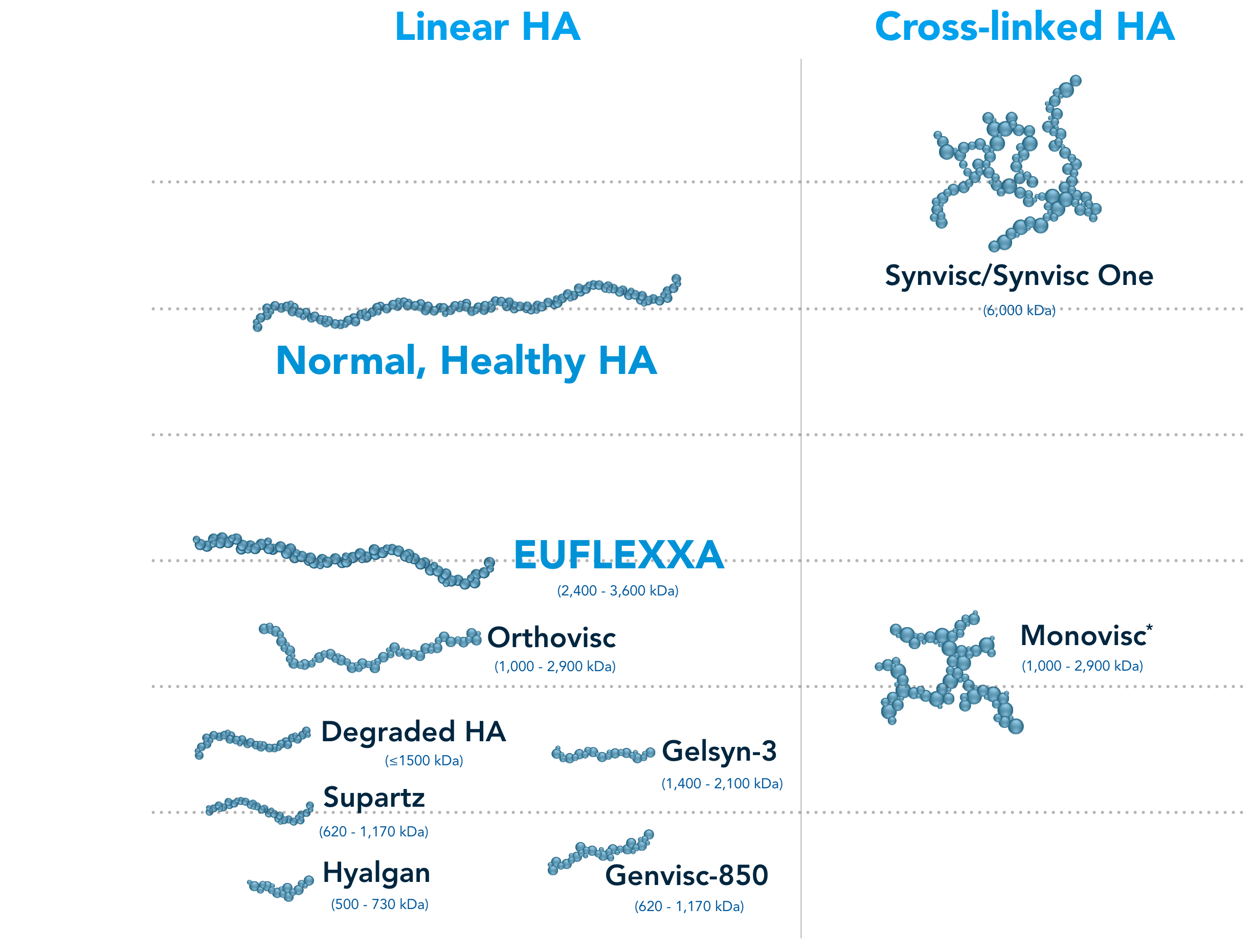
EUFLEXXA most closely resembles healthy, human HA2,6-11
This table does not represent all HA products.
Only HA products above a certain market share represented here.1
Durolane, a cross-linked HA, does not report their molecular weight.13
Gel-One, a cross-linked HA, does not report their molecular weight.15
*Same grade and specification of HA that is used in Orthovisc.2-4
ORTHOVISC and MONOVISC are registered trademarks of Anika Therapeutics, Inc. Gelsyn-3 and DUROLANE are trademarks of Bioventus LLC. Synvisc and Synvisc-One are trademarks of Genzyme Corporation. HYALGAN is a registered trademark of Fidia Farmaceutici S.p.A. SUPARTZ, SUPARTZ FX, and Gel-One are trademarks
of Seikagaku Corp.
The information set forth herein is presented to provide reference to various characteristics that were examined for the products in the HA class for treatment of OA knee pain. This information does not suggest or imply in any manner that any of the characteristics of each HA product, alone or in combination, are superior over another. Nor is it intended to imply that the products mentioned have comparable safety or efficacy, as head-to-head clinical data are not available for all.
Resources
& Coverage
Find helpful resources and coverage to
support your practice and your patients.
- Rolling 12 month average of IQVIA claims data based on unique patients (December 2022).
- EUFLEXXA [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.
- Altman R, et al. Anti-inflammatory effects of intra-articular hyaluronic acid: a systematic review. Cartilage. 2019;10(1):43-52.
- Altman RD, Manjoo A, Fierlinger A, et al. The mechanism
of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disor. 2015;16:321. - Yang C, Cao M, Liu H, et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 2012;287(51):43094-43107.
- Nicholls M, et al. A comparison between rheological properties of intra-articular hyaluronic acid preparations and reported human synovial fluid. Adv Ther. 2018;35:523-530.
- Orthovisc [package insert]. Woburn, MA: Anika Therapeutics, Inc; 2005.
- Supartz [package insert]. Durham, NC: Bioventus LLC; 2012.
- Gelsyn-3 [package insert]. Durham, NC: Bioventus LLC; 2016.
- Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc; 2011.
- Genvisc-850 [package insert]. Doylestown, PA: OrthogenRx; 2016.
- Supartz FX [package insert]. Durham, NC: Bioventus LLC; 2015.
- Durolane [package insert]. Durham, NC: Bioventus LLC.
- Monovisc. Summary of safety and effectiveness data. Monovisc Injectable Intra-articular Device-P090031.
- Gel-One [package insert]. Warsaw, IN.
- Synvisc [package insert]. Cambridge, MA: Genzyme Corp; 2010.
- Synvisc-One [package insert]. Cambridge, MA; Genzyme Corp; 2010.
