Dual-acting EUFLEXXA: Robust study design. Statistically significant results.2,3
The efficacy and safety of EUFLEXXA was studied across 3 robust clinical trials2,3
Compared to Synvisc, EUFLEXXA delivered:
Primary Endpoint
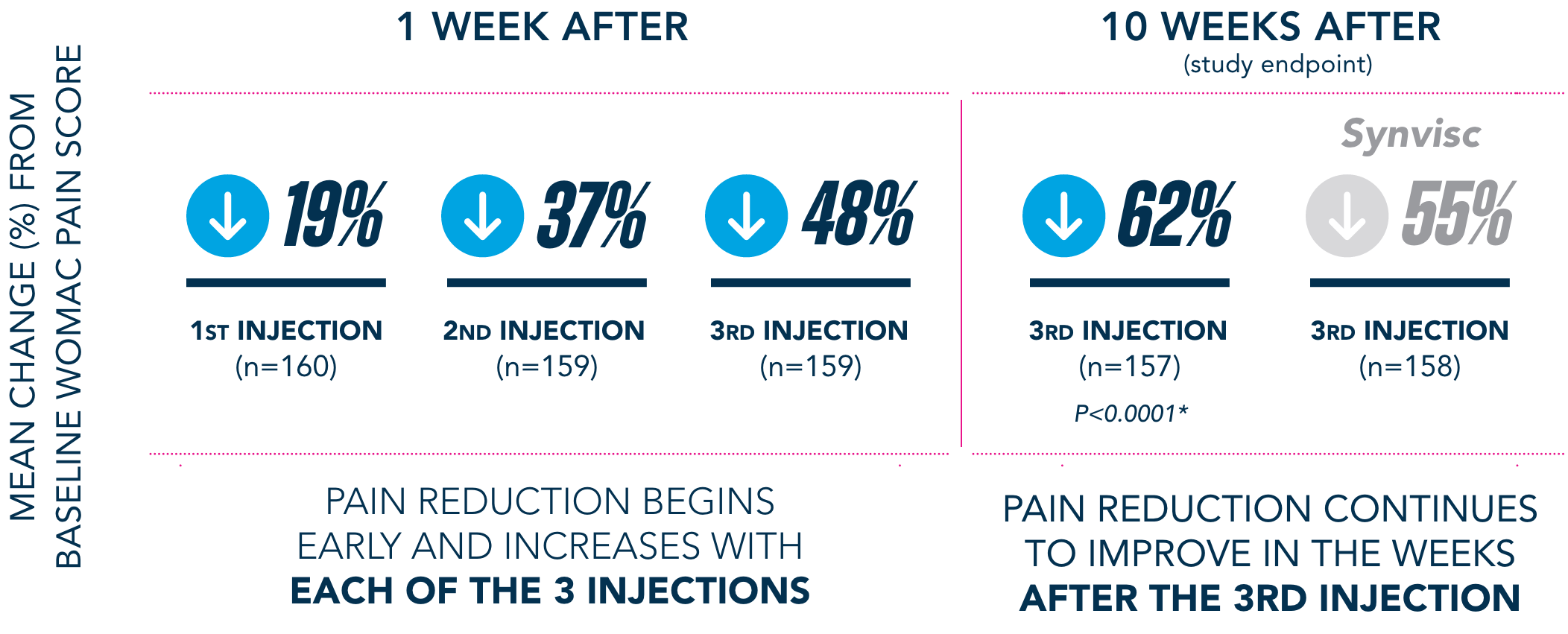
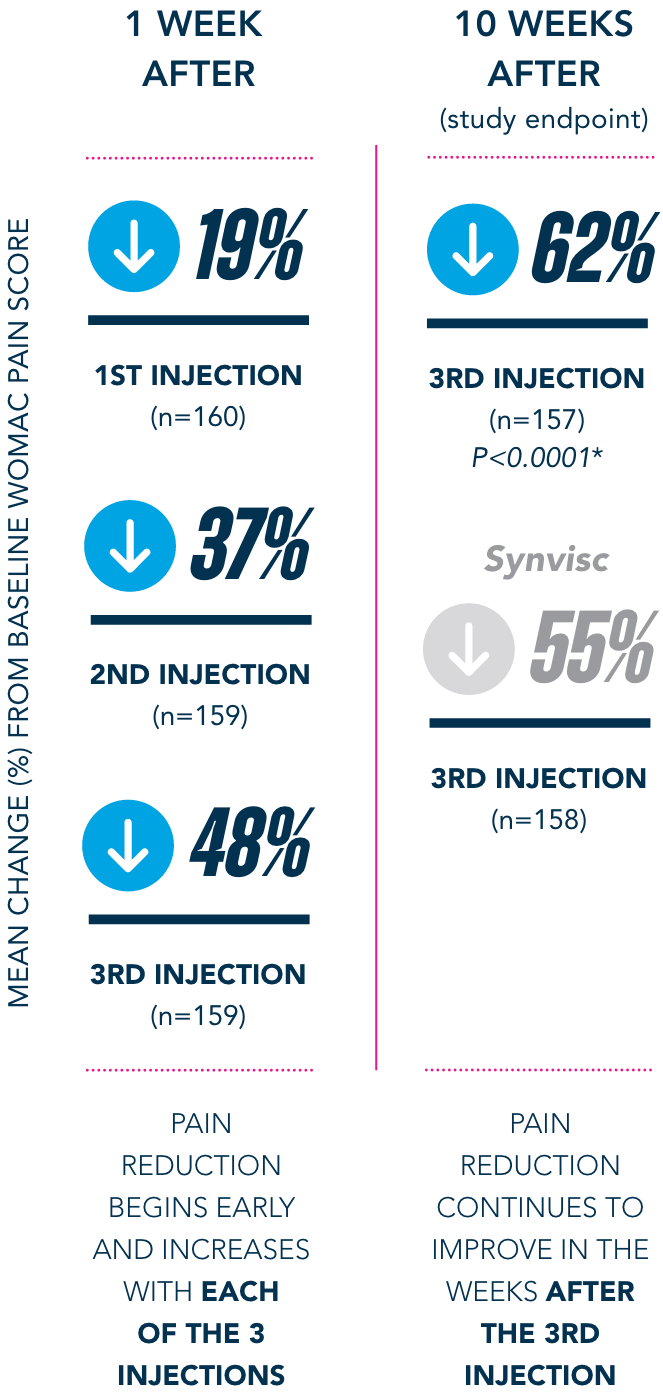

†Pain-free is defined as symptom-free for the 5 WOMAC pain questions (with average visual analog scale [VAS] scores of <20mm).
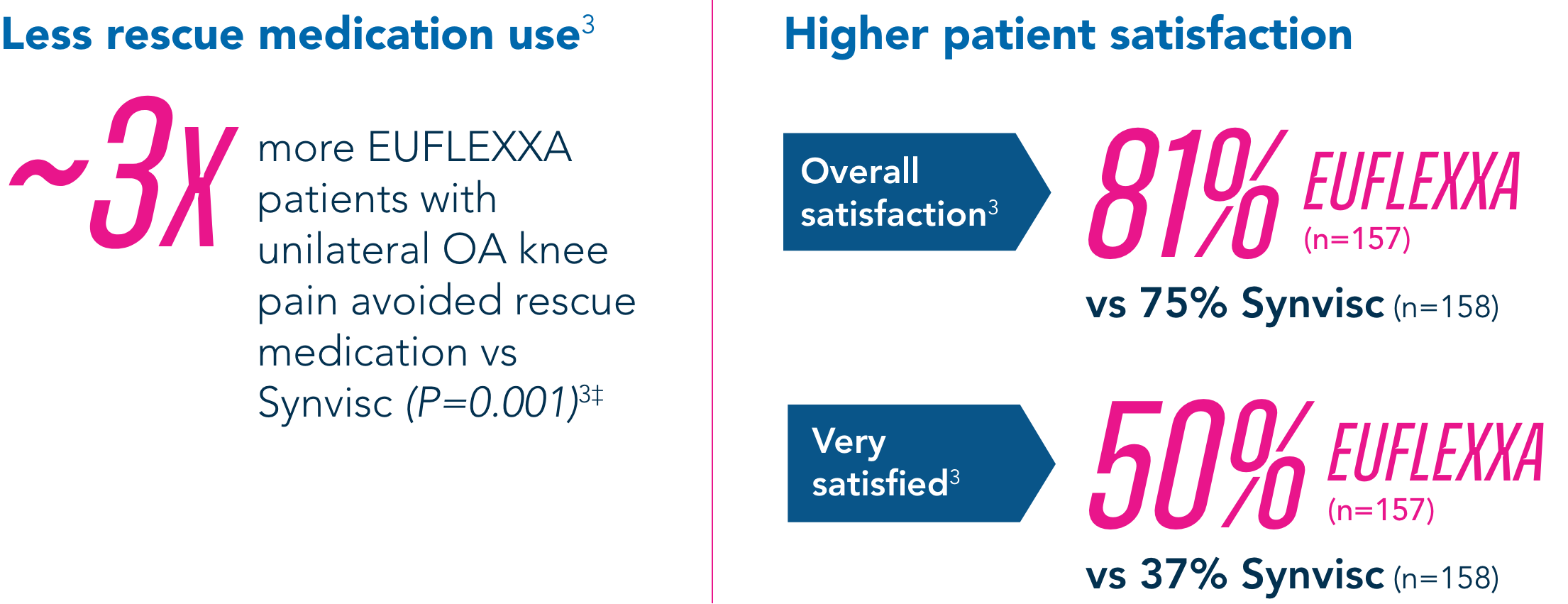
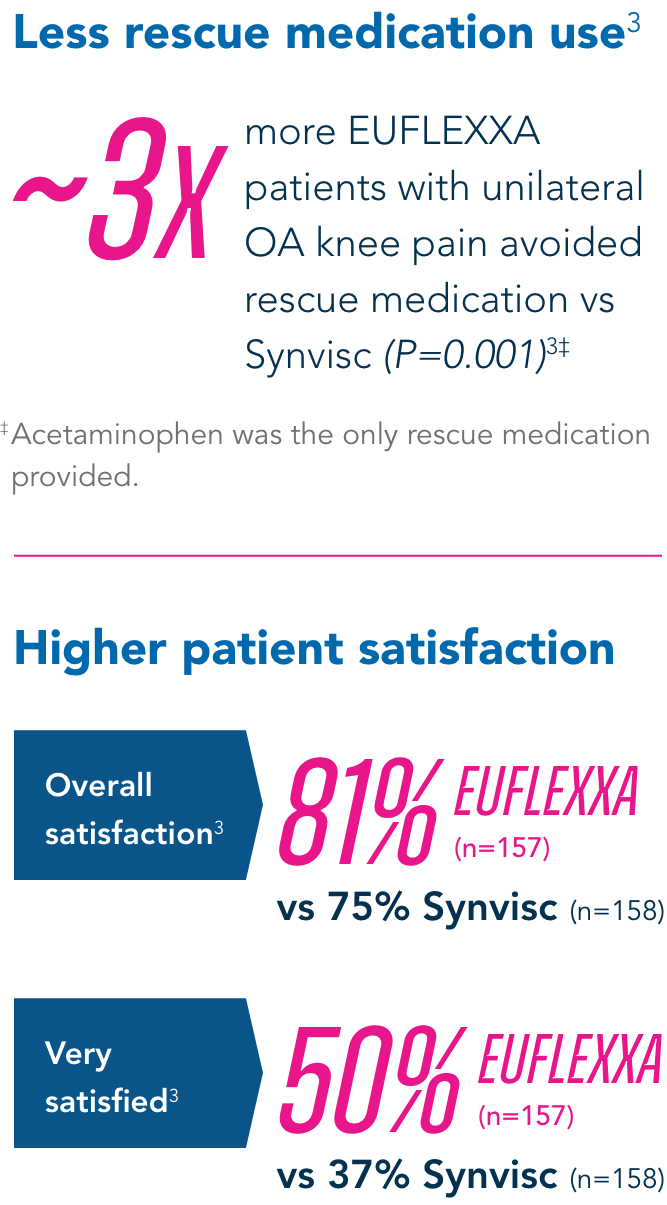
Secondary Endpoints
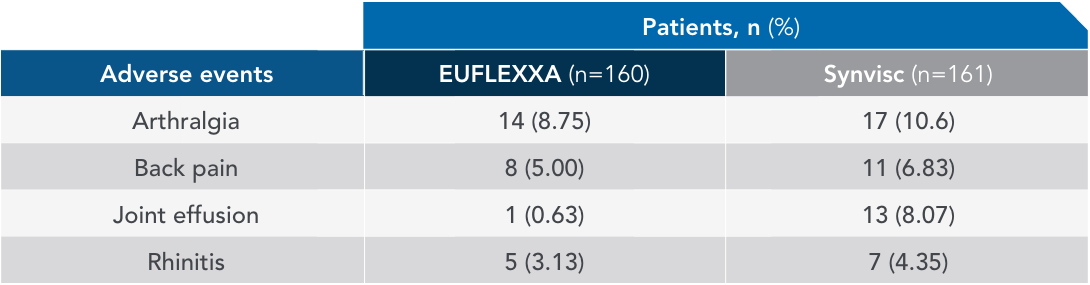
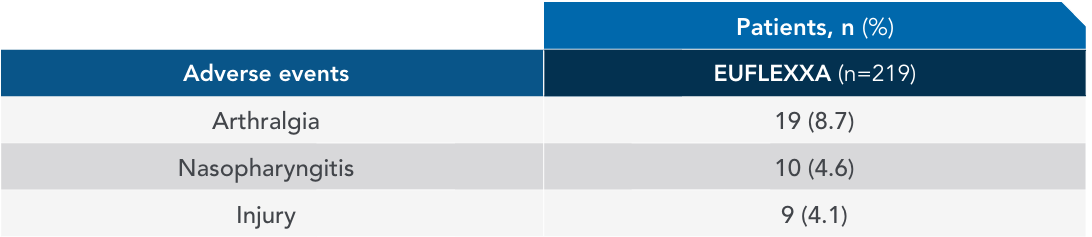
Most common adverse events (AEs) in 12-week pivotal trial vs Synvisc2,3
Compared to the control group, EUFLEXXA delivered:
Primary Endpoint
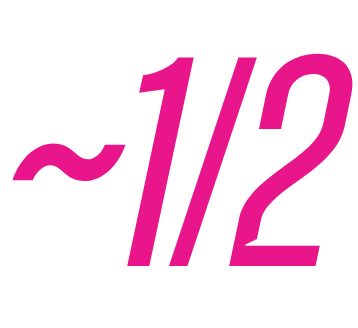
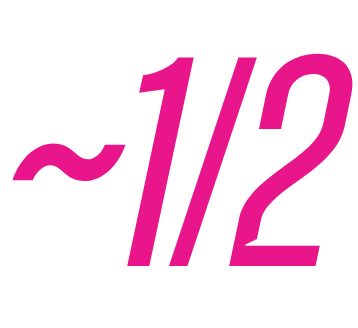
of EUFLEXXA patients were PAIN-FREE at 6 months2,4
Pain-free (<20 mm) was determined at week 26 using the 100 mm VAS based on the 50-foot walk test.
Secondary Endpoint
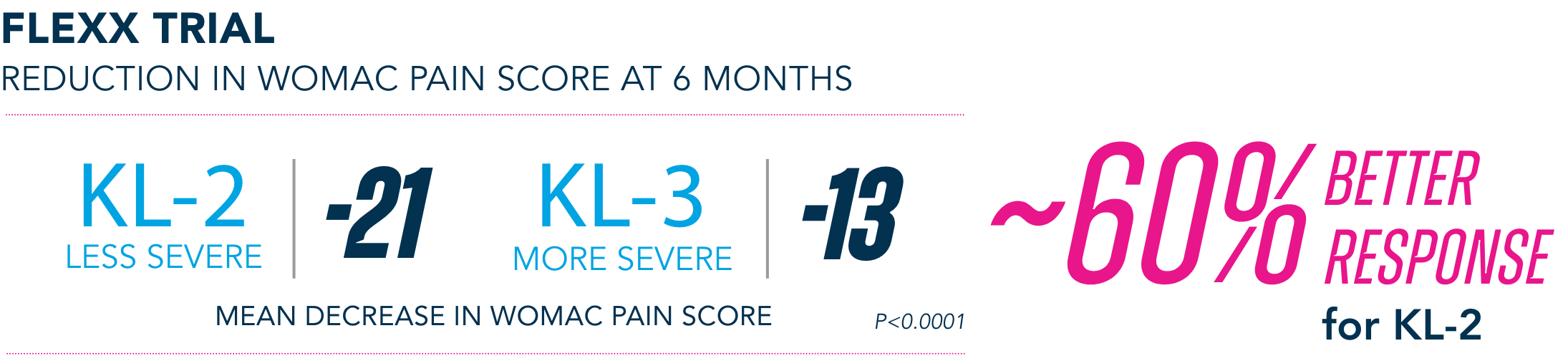
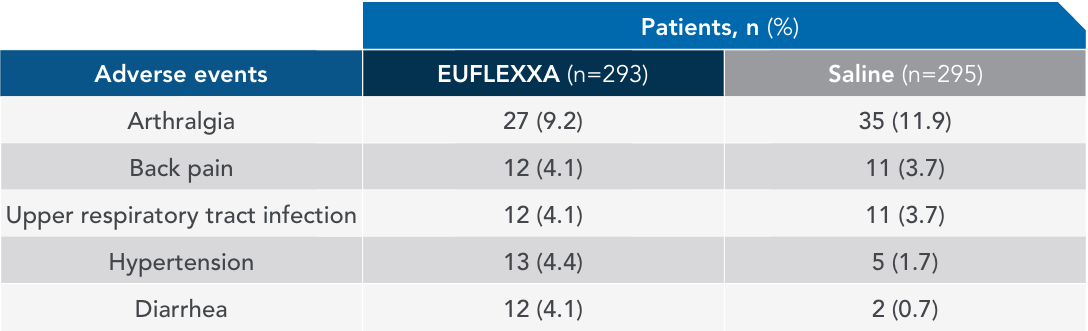
Most common AEs in 26-week FLEXX trial2
the treatment groups
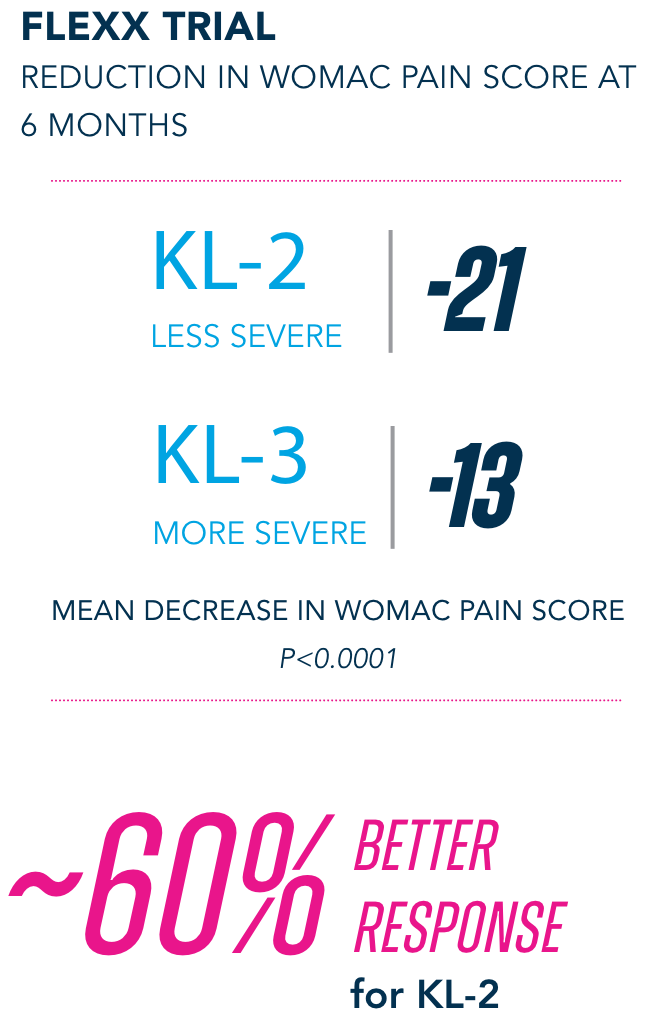
Primary Endpoint
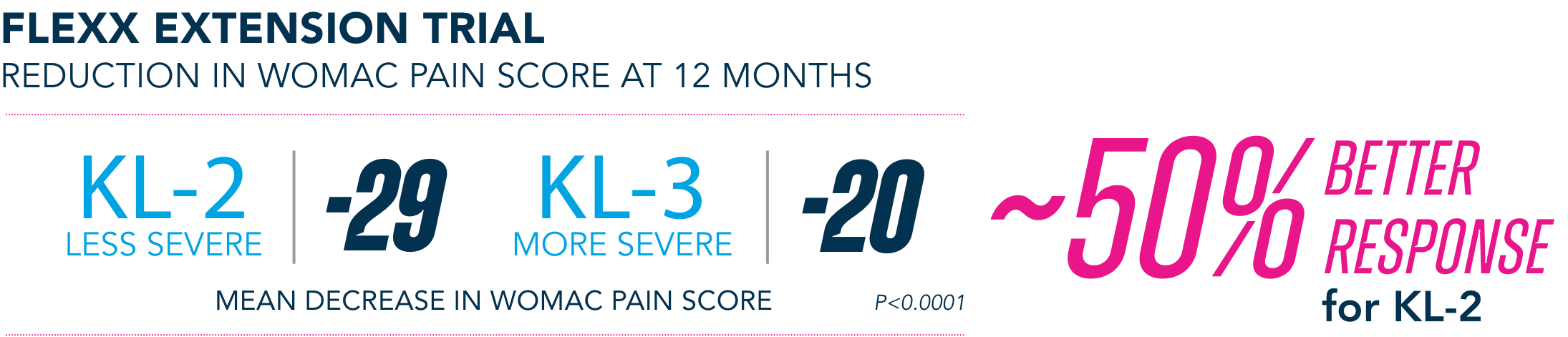
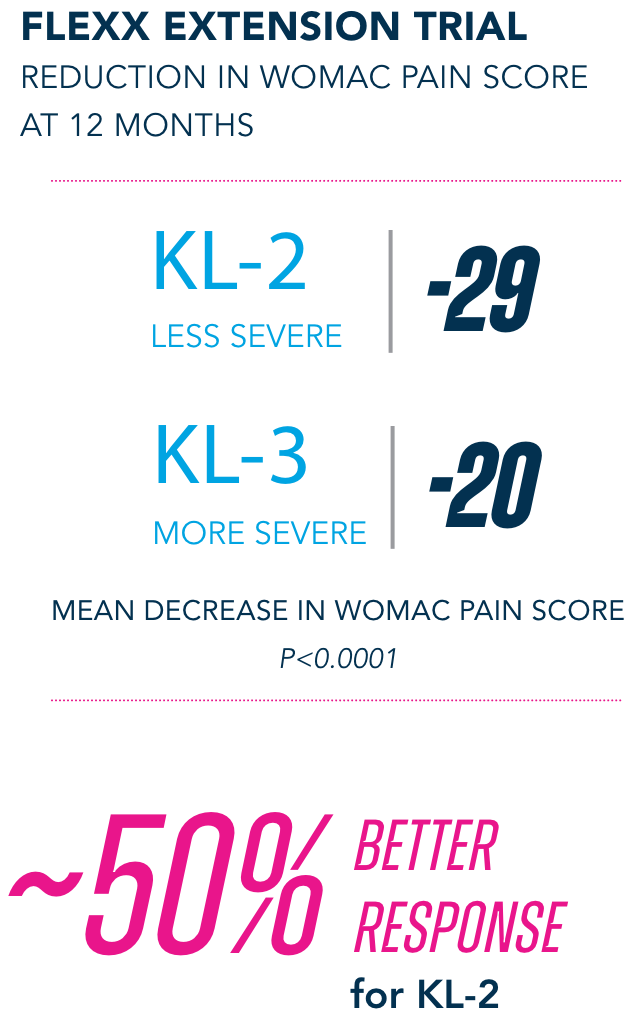
Post-hoc analyses are limited by their post-hoc design and thus prone to increased bias. Such analyses are exploratory and should not be used to infer causative relationships between variables.
Most common AEs in 26-week FLEXX Extension trial2
among the treatment groups
§KL=Kellgren-Lawrence grading scale; KL grades are determined radiographically. KL-2 (n=235), KL-3 (n=353).
¶All numbers rounded to the nearest whole number.
- Rolling 12 month average of IQVIA claims data based on unique patients (December 2022).
- EUFLEXXA [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.
- Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(2):154-162.
- Altman RD, Rosen JE, Bloch DA, et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX Trial). Semin Arthritis Rheum. 2009;39(1):1-9.
- Altman RD, Rosen JE, Bloch DA, et al. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label Extension Study of the FLEXX Trial. Osteoarthritis Cartilage. 2011;19(10):1169-1175.
